2018 Waec Gce Chemistry Objective and Essay Questions and Answers is available
Keep Refreshing This page to see More answers
CHEMISTRY OBJ:
1-10: CCDBACBABA
11-20: BDDDCDBAAA
21-30: CAADCCCAAB
31-40: CBBCABCDBC
41-50: BACCAABACC
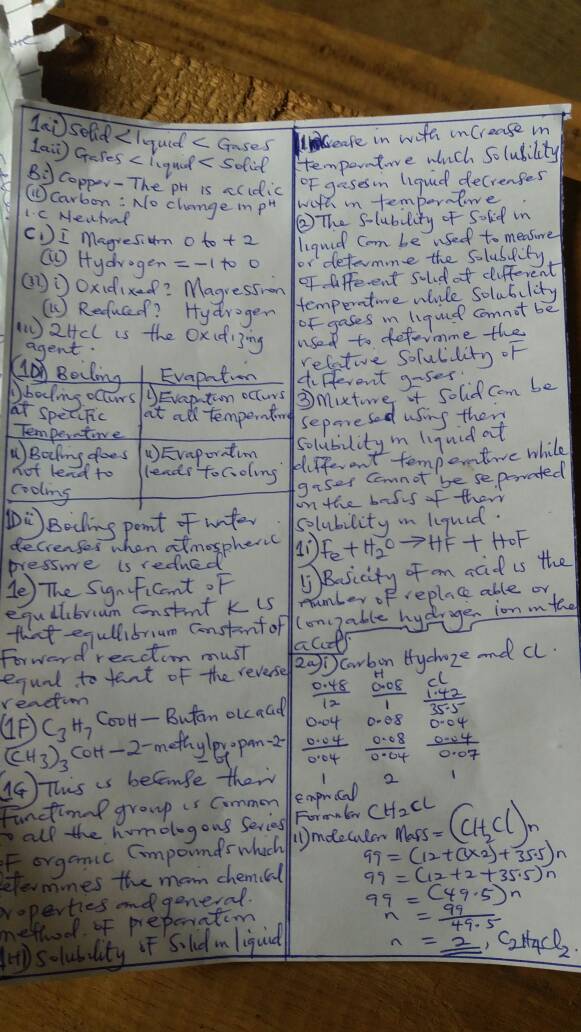
(1a)
Gas > liquid > Solid
(1aii)
Solid > liquid > gas
(1bi)
PH>7
(1bii)
PH>7
(1ci)
(I) Magnesium from 0 to +2
(II) Hydrogen changes from 1 to 0
(1cii)
I ---> Magnesium is oxidized
II ---> Hyfrogen is reduced
(1ciii)
HCL
(1di)
(i) Boiling occurs throughout the entire volume while evaporation occurs at the surface
(ii)It involves the formation of bubbles while evaporation does not form bubbles
(1dii)
It decreases the boiling point as the boiling point of water is proportional to the magnitude of atmosphere pressure.
(1e)
It dictates the rate of reaction and also optimize the amount of product formed.
(1f)
C3H7CooH ---> Butanoic acid
(CH3)3COH ---> Butan-1-ol
(1g)
Functional groups are responsible for their different chemical behavior or properties so that organic compounds with smaller functional group would behave similarly.
(1h)
Solids in liquid:
(i) It depends on the nature of temperature changes or exothermic or endothermic as affect by the temperature of the liquid.
(ii) The surface area of solid is lesser, they dissolves less as compound to gases in the same volume.
(iii) Pressure does not affect the solubility of the solids
Gas in liquid:
(i) It depends on the average kinetic energy as affect by temperature of the liquid.
(ii) The surface area of gases are higher so they dissolve more in a given volume of water.
(iii) The solubility of gases is a direct consequences of pressure changes due to the partial pressure of the gas molecules.
(2i)
C = 0.48 H = 0.8 Cl = 1.42
H
C = 1 H = 1 Cl = 35.5
C = 0.48/12
H = 0.08/1
Cl = 1.42/35.5
0.04/0.04 , 0.08/0.04 , 0.04/0.04
Therefore CH2CL
The electrical formular = CH2CL
(2aii) The molar mass of the compound is 99
Hence (CH2CL)n = 99
(12 + 2C1) + 35.5)n = 99
12 + 2 + 35.5
(495)n = 99
Therefore n = 2
Hence the molar formula = (CH2CL)2
C2H4CL2
(2b)
(I) it is soluble in water and in other polar solvents.
(II) Nacl(s) when dissolve in water ionized to form Na+ and Cl-
(III) the aqueous solution of Nacl(s) can be use as n electrolyte
(2ci) DRAW THE DIAGRAM
(2cii)
(i)Eq1- actuation energy for in catalyzed reaction
(ii)Eq2 - actuation energy for catalyzed reaction
(3ai)
When potassium chloride dissolves in water, the bond in the solid particle breaks thereby increasing the kinetic energy of the particle to move. Particles in solid state have restricted or static movement unlike those in the form of ions or gases.
(3aii)
It is endothermic because it involves bond breaking and heat is absorbed in the process.
(3bi)
An underground iron pipe is less likely to erode if it is bonded at intervals with magnesium rod because the magnesium is more electro positive thereby absorbing the water or little oxynegn it is exposed to. In addition, corrosion is influenced by the presence of oxygen and water, therefore underground iron pipe will be exposed to little oxygen and water.
(3bii)
(i) First, conversion of Iron II to Iron III
Fe²+(aq) + e- --->Fe³+(aq)
(ii) Exposure to oxygen and water vapour.
4Fe(s) + 3O2(g) + xH2O(l) --> 2Fe2O3 xH2O
Iron rod Iron rust
(3biii)
The iron in water will be oxidized since there is dissolved oxygen and atmospheric oxygen & a brown deposit of Iron occurred.
(3ci)
A spontaneous reaction is a reaction that can exist freely on its own without any external factor. A reaction is said to be spontaneous if ΔG, is the Gribb's free energy is negative. ΔG = -ve
An example is rusting of Iron.
(3cii)
(i) When Gribb's free energy is negative.
(ii) When Entropy ie Δs must be positive as the total entropy must increase.
(3Ciii)
This is because sodium is more electropositive than calcium. Also the heat evolved in the reaction with sodium is far greater than the heat of solution released with calcium and water at the same temperature.
(3civ)
2Na(s) + 2H2O(l) ---> 2NaOH(aq) + H2(g)
Ca(s) + 2H2O(l) ---> Ca(OH)2 + H2(g)
(3d)
Mass of lead (ii) trioxocarbonate iv
PbCO3
= 207 + 12 + 3(16)
207 + 12 + 48 = 267g
1mole of PbCO3 contain 1 mole of Pb
267g of PbCO3 = 207g of Pb
Xg of PbCO3 = 35.0g of P
X = 267×35/207 = 45.1g
=45.1g of PbCO3
(3e)
(i) Covalent bond
(ii) Ionic bond
No4a
Town Water Supplies | Water Treatment
Below are the processes which water may be subjected to, in its purification at the water works before being distributed for human use:.
1. Coagulation, Flocculation, and sedimentation - These are the processes of rapid mixing of chemicals known as coagulants to make the solid particles in the water clump together (coagulation), and then the gentle mixing to form larger groups of particles known as floc (flocculation). Alum (aluminium sulphate), polyaluminium chloride and a group of chemicals known as polyelectrolytes (these are polymers with ionizable groups that can dissociate in solution, leaving ions of one sign bound to the chain and counterions in solution) are the materials currently used for this purpose. This thicker, denser floc floats down by gravity and settle out of the water in large tanks (sedimentation) or is removed during filtration
2. Filtration - In this stage the remaining floc, other chemicals and physical impurities, and most of the biological impurities (bacteria, etc.) are removed. The water flows by gravity through filters. There are different types of filters which may be employed. A type is the tank-type pressure filter which consist of: oxidizing filters; activated carbon filters; and dual or multi-media filters.
Oxidizing filters - these use a medium treated with oxides of manganese as a source of oxygen to oxidize and precipitate Iron, manganese, hydrogen sulphide, and others. Activated carbon filters - these are similar to ion exchange resins in density and porosity. They absorb low molecular weight organics and reduce chloride or other halogens from water, but do not remove any salts.
They must be change periodically to avoid bacterial growth, but are not easily reactivated in the field. Dual or multi-media filters - these remove suspended solids to as low as 20 microns in size, but not dissolved solids. The top layer is coarse anthracite followed by fine sand. Note: the type of filters used depends primarily on the nature of the water.
3. Disinfection or Sterilization - The destruction of disease-causing organisms in the raw and treated water through the addition of the chemical, chlorine is the most important step in the water treatment process. The chemical is added to water at different points in the treatment process. When chlorine is added to the raw water as it enters the plant, the process is known as pre-chlorination.
When it is added after filtration, it is known as post-chlorination. Additional chlorine can be added when the levels of bacteria are high, through a process known as super chlorina- tion. Sulphur(IV) oxide is then added to the water- it combines with the excess chlorine to reduce the chlorine residual to an acceptable level before the ammoniation stage. Sterilization of water can also be done using ozone, but this is very expensive
5. Ammoniation - Ammonia is added to combine with the remaining chlorine. This stabilizes the chlorine so that it remains dissolved in the treated water for longer periods of time, keeping the water safe during its long trip through the distribution system. Ammoniation also prevents minute amounts of chlorine from evaporating out of drinking water (causing smells).
6. Lime Treatment (Ca(OH) 2 ) - The addition of lime and soda ash (Na 2 CO 3 ) reduces the level of calcium and magnesium in the water, and is referred to as “lime softening”. The purpose of lime softening is to remove hardness, and then clarify the water and improve its taste
7. pH Adjustment - Municipal water may be pH adjusted to a pH of an approximately 7.5 to 8.0 to prevent corrosion of water pipes, particularly to prevent dissolution of lead into the water supply. In the case of excessive alkalinity, the pH may be reduced by the addition of CO 2 . Also, pH adjustment is important because certain chemicals, membranes, ion exchange resins and other materials used in water treatment are sensitive to specific pH conditions; and is to prevent acid corrosion in boiler feed water by adjusting the pH to be between 8.3 and 9.0 .
Other Chemicals used in Water Treatment Processes: A. Dispersants Dispersants are added when scaling may be expected due to concentration of specific ions in the stream. Dispersants disrupt the scale formation, preventing growth of precipitated crystals. B. Sequestering (chelating) Agents Sequestering agents, e.g., polymetaphosphate, (NaPO3 )n and tetrasodium diphosphate, Na4 P 2 O7 are used to prevent the negative effects of hardness, preventing the deposition of Ca, Mg, Fe, Mn and Al. C. Oxidizing Agents Oxidizing agents have two distinct functions: as a biocide, or to neutralize reducing agents. Example, potassium permanganate - potassium permanganate (KMnO 4 ) is a strong oxidizing agent used in many bleaching applications.
It will oxidize most organic compounds and is often used to oxidize ferrous iron to ferric for precipitation and filtration. D. Reducing Agents Reducing agents, like sodium metabisulphite (Na2 S 2 O 5 ), are added to neutralize oxidizing agents such as chlorine or ozone. In membrane and ion exchange systems, they prevent the degradation of certain membranes or resins, which are sensitive to oxidizing agents.
(5ai)
(i)Carbon (II) oxide
(ii) Carbon (iv) oxide
(5aii)
It is because there is increasing surface area of the broken coal than in the lumps of coal
(5aiii)
CH4
(5aiv)
Coke
(5bi)
SO2
(5bii)
MnO2
(5biii)
Sodium oxide
(5biv)
Aluminium oxide
(5bv)
NO2 gas
(5ci)
Cl2+FeCl----->FeCl3
(5cii)
Redox reaction
(5ciii)
Iron(ii) ion is oxidized to iron(iii) ion
ie Fe2+ --> Fe3+
(5di)
A = Delivery tube
B = Calcium oxide
(5dii)
Ca(OH)2+2NH4Cl --> CaCl2+2NH4.H2O
(5diii)
Ammonia gas
(5div)
The gas is less dense than air
(5dv)
(I) B is used to drive the gas
(II) Upward delivery
(5e)
Petrol
2018 #Waec #Gce Chemistry Objective and Essay #Questions and #answers now available, To get all questions and answers for Chemistry Objective and Essay .
9Jatechs deliver it answers at midnight to her subscribe.
Subjects for Tuesday, 18th September, 2018
Chemistry 2 (Essay) >> 9.30am – 11.30am
Chemistry 1 (Objective) >> 11.30am – 12.30pm
Procedure on How To Subscribe for 2018 Waec Chemistry Objective and Essay Questions and Answers
We only Run our Waec Gce Packages on Whatsappbecause it is the fastest means of sending messages and photos online.
Answers delivery via whatsapp for Chemistry Objective and Essay Questions and Answers Cost N600 MTN recharge card.
ALSO SEE>>2018 Waec Gce Chemistry Practical Questions and Answers | Waec Gce Exam Runs
First Procedure on how to Subscribe
To subscribe for Chemistry Questions and Answers send the card pin, Subject name(Chemistry Objective and Essay Questions and answers ) and your phone number Via SMS or Via WhatsApp To 08065889844
ALSO SEE>>2018 Waec Gce CRS Objective and Essay Questions and Answers | Waec Gce Exam Expo
Note: please do not call our number, simply send message on whatsapp or using SMS
For More Info Whatsapp 08065889844
2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for .2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for Chemistry Objective and Essay .
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
Also see the 2018 Waec Gce Biology Practical Questions and Answers.
2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for .2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for Chemistry Objective and Essay .
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
ALSO SEE>>2018 Waec Gce Physical and Practical Geography Questions and Answers | Waec Gce Expo
2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for .2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for Chemistry Objective and Essay .
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
Keep Refreshing This page to see More answers
CHEMISTRY OBJ:
1-10: CCDBACBABA
11-20: BDDDCDBAAA
21-30: CAADCCCAAB
31-40: CBBCABCDBC
41-50: BACCAABACC
ANSWERS NUMBER 1,2 AND 4 IN THE PHOTO BELOW.
(1a)
Gas > liquid > Solid
(1aii)
Solid > liquid > gas
(1bi)
PH>7
(1bii)
PH>7
(1ci)
(I) Magnesium from 0 to +2
(II) Hydrogen changes from 1 to 0
(1cii)
I ---> Magnesium is oxidized
II ---> Hyfrogen is reduced
(1ciii)
HCL
(1di)
(i) Boiling occurs throughout the entire volume while evaporation occurs at the surface
(ii)It involves the formation of bubbles while evaporation does not form bubbles
(1dii)
It decreases the boiling point as the boiling point of water is proportional to the magnitude of atmosphere pressure.
(1e)
It dictates the rate of reaction and also optimize the amount of product formed.
(1f)
C3H7CooH ---> Butanoic acid
(CH3)3COH ---> Butan-1-ol
(1g)
Functional groups are responsible for their different chemical behavior or properties so that organic compounds with smaller functional group would behave similarly.
(1h)
Solids in liquid:
(i) It depends on the nature of temperature changes or exothermic or endothermic as affect by the temperature of the liquid.
(ii) The surface area of solid is lesser, they dissolves less as compound to gases in the same volume.
(iii) Pressure does not affect the solubility of the solids
Gas in liquid:
(i) It depends on the average kinetic energy as affect by temperature of the liquid.
(ii) The surface area of gases are higher so they dissolve more in a given volume of water.
(iii) The solubility of gases is a direct consequences of pressure changes due to the partial pressure of the gas molecules.
(2i)
C = 0.48 H = 0.8 Cl = 1.42
H
C = 1 H = 1 Cl = 35.5
C = 0.48/12
H = 0.08/1
Cl = 1.42/35.5
0.04/0.04 , 0.08/0.04 , 0.04/0.04
Therefore CH2CL
The electrical formular = CH2CL
(2aii) The molar mass of the compound is 99
Hence (CH2CL)n = 99
(12 + 2C1) + 35.5)n = 99
12 + 2 + 35.5
(495)n = 99
Therefore n = 2
Hence the molar formula = (CH2CL)2
C2H4CL2
(2b)
(I) it is soluble in water and in other polar solvents.
(II) Nacl(s) when dissolve in water ionized to form Na+ and Cl-
(III) the aqueous solution of Nacl(s) can be use as n electrolyte
(2ci) DRAW THE DIAGRAM
(2cii)
(i)Eq1- actuation energy for in catalyzed reaction
(ii)Eq2 - actuation energy for catalyzed reaction
(3ai)
When potassium chloride dissolves in water, the bond in the solid particle breaks thereby increasing the kinetic energy of the particle to move. Particles in solid state have restricted or static movement unlike those in the form of ions or gases.
(3aii)
It is endothermic because it involves bond breaking and heat is absorbed in the process.
(3bi)
An underground iron pipe is less likely to erode if it is bonded at intervals with magnesium rod because the magnesium is more electro positive thereby absorbing the water or little oxynegn it is exposed to. In addition, corrosion is influenced by the presence of oxygen and water, therefore underground iron pipe will be exposed to little oxygen and water.
(3bii)
(i) First, conversion of Iron II to Iron III
Fe²+(aq) + e- --->Fe³+(aq)
(ii) Exposure to oxygen and water vapour.
4Fe(s) + 3O2(g) + xH2O(l) --> 2Fe2O3 xH2O
Iron rod Iron rust
(3biii)
The iron in water will be oxidized since there is dissolved oxygen and atmospheric oxygen & a brown deposit of Iron occurred.
(3ci)
A spontaneous reaction is a reaction that can exist freely on its own without any external factor. A reaction is said to be spontaneous if ΔG, is the Gribb's free energy is negative. ΔG = -ve
An example is rusting of Iron.
(3cii)
(i) When Gribb's free energy is negative.
(ii) When Entropy ie Δs must be positive as the total entropy must increase.
(3Ciii)
This is because sodium is more electropositive than calcium. Also the heat evolved in the reaction with sodium is far greater than the heat of solution released with calcium and water at the same temperature.
(3civ)
2Na(s) + 2H2O(l) ---> 2NaOH(aq) + H2(g)
Ca(s) + 2H2O(l) ---> Ca(OH)2 + H2(g)
(3d)
Mass of lead (ii) trioxocarbonate iv
PbCO3
= 207 + 12 + 3(16)
207 + 12 + 48 = 267g
1mole of PbCO3 contain 1 mole of Pb
267g of PbCO3 = 207g of Pb
Xg of PbCO3 = 35.0g of P
X = 267×35/207 = 45.1g
=45.1g of PbCO3
(3e)
(i) Covalent bond
(ii) Ionic bond
No4a
Town Water Supplies | Water Treatment
Below are the processes which water may be subjected to, in its purification at the water works before being distributed for human use:.
1. Coagulation, Flocculation, and sedimentation - These are the processes of rapid mixing of chemicals known as coagulants to make the solid particles in the water clump together (coagulation), and then the gentle mixing to form larger groups of particles known as floc (flocculation). Alum (aluminium sulphate), polyaluminium chloride and a group of chemicals known as polyelectrolytes (these are polymers with ionizable groups that can dissociate in solution, leaving ions of one sign bound to the chain and counterions in solution) are the materials currently used for this purpose. This thicker, denser floc floats down by gravity and settle out of the water in large tanks (sedimentation) or is removed during filtration
2. Filtration - In this stage the remaining floc, other chemicals and physical impurities, and most of the biological impurities (bacteria, etc.) are removed. The water flows by gravity through filters. There are different types of filters which may be employed. A type is the tank-type pressure filter which consist of: oxidizing filters; activated carbon filters; and dual or multi-media filters.
Oxidizing filters - these use a medium treated with oxides of manganese as a source of oxygen to oxidize and precipitate Iron, manganese, hydrogen sulphide, and others. Activated carbon filters - these are similar to ion exchange resins in density and porosity. They absorb low molecular weight organics and reduce chloride or other halogens from water, but do not remove any salts.
They must be change periodically to avoid bacterial growth, but are not easily reactivated in the field. Dual or multi-media filters - these remove suspended solids to as low as 20 microns in size, but not dissolved solids. The top layer is coarse anthracite followed by fine sand. Note: the type of filters used depends primarily on the nature of the water.
3. Disinfection or Sterilization - The destruction of disease-causing organisms in the raw and treated water through the addition of the chemical, chlorine is the most important step in the water treatment process. The chemical is added to water at different points in the treatment process. When chlorine is added to the raw water as it enters the plant, the process is known as pre-chlorination.
When it is added after filtration, it is known as post-chlorination. Additional chlorine can be added when the levels of bacteria are high, through a process known as super chlorina- tion. Sulphur(IV) oxide is then added to the water- it combines with the excess chlorine to reduce the chlorine residual to an acceptable level before the ammoniation stage. Sterilization of water can also be done using ozone, but this is very expensive
5. Ammoniation - Ammonia is added to combine with the remaining chlorine. This stabilizes the chlorine so that it remains dissolved in the treated water for longer periods of time, keeping the water safe during its long trip through the distribution system. Ammoniation also prevents minute amounts of chlorine from evaporating out of drinking water (causing smells).
6. Lime Treatment (Ca(OH) 2 ) - The addition of lime and soda ash (Na 2 CO 3 ) reduces the level of calcium and magnesium in the water, and is referred to as “lime softening”. The purpose of lime softening is to remove hardness, and then clarify the water and improve its taste
7. pH Adjustment - Municipal water may be pH adjusted to a pH of an approximately 7.5 to 8.0 to prevent corrosion of water pipes, particularly to prevent dissolution of lead into the water supply. In the case of excessive alkalinity, the pH may be reduced by the addition of CO 2 . Also, pH adjustment is important because certain chemicals, membranes, ion exchange resins and other materials used in water treatment are sensitive to specific pH conditions; and is to prevent acid corrosion in boiler feed water by adjusting the pH to be between 8.3 and 9.0 .
Other Chemicals used in Water Treatment Processes: A. Dispersants Dispersants are added when scaling may be expected due to concentration of specific ions in the stream. Dispersants disrupt the scale formation, preventing growth of precipitated crystals. B. Sequestering (chelating) Agents Sequestering agents, e.g., polymetaphosphate, (NaPO3 )n and tetrasodium diphosphate, Na4 P 2 O7 are used to prevent the negative effects of hardness, preventing the deposition of Ca, Mg, Fe, Mn and Al. C. Oxidizing Agents Oxidizing agents have two distinct functions: as a biocide, or to neutralize reducing agents. Example, potassium permanganate - potassium permanganate (KMnO 4 ) is a strong oxidizing agent used in many bleaching applications.
It will oxidize most organic compounds and is often used to oxidize ferrous iron to ferric for precipitation and filtration. D. Reducing Agents Reducing agents, like sodium metabisulphite (Na2 S 2 O 5 ), are added to neutralize oxidizing agents such as chlorine or ozone. In membrane and ion exchange systems, they prevent the degradation of certain membranes or resins, which are sensitive to oxidizing agents.
(5ai)
(i)Carbon (II) oxide
(ii) Carbon (iv) oxide
(5aii)
It is because there is increasing surface area of the broken coal than in the lumps of coal
(5aiii)
CH4
(5aiv)
Coke
(5bi)
SO2
(5bii)
MnO2
(5biii)
Sodium oxide
(5biv)
Aluminium oxide
(5bv)
NO2 gas
(5ci)
Cl2+FeCl----->FeCl3
(5cii)
Redox reaction
(5ciii)
Iron(ii) ion is oxidized to iron(iii) ion
ie Fe2+ --> Fe3+
(5di)
A = Delivery tube
B = Calcium oxide
(5dii)
Ca(OH)2+2NH4Cl --> CaCl2+2NH4.H2O
(5diii)
Ammonia gas
(5div)
The gas is less dense than air
(5dv)
(I) B is used to drive the gas
(II) Upward delivery
(5e)
Petrol
2018 #Waec #Gce Chemistry Objective and Essay #Questions and #answers now available, To get all questions and answers for Chemistry Objective and Essay .
9Jatechs deliver it answers at midnight to her subscribe.
Subjects for Tuesday, 18th September, 2018
Chemistry 2 (Essay) >> 9.30am – 11.30am
Chemistry 1 (Objective) >> 11.30am – 12.30pm
9Jatechs provide you a forum for all exam runs named Waec Exam Forum and Neco Exams Which enable you to see all previous and current waec(gce) and Neco(Gce) Answers and questions.
2018 Waec Gce Chemistry Objective and Essay Answers will be deliver to all 9Jatechs Subscribers at night before the exam day, you can also get free exam Expo on our portal(i. e we are going to post answers 1hour after the exam start) just like we did during the previous Mathematics Objective and Theory exam
Procedure on How To Subscribe for 2018 Waec Chemistry Objective and Essay Questions and Answers
We only Run our Waec Gce Packages on Whatsappbecause it is the fastest means of sending messages and photos online.
Answers delivery via whatsapp for Chemistry Objective and Essay Questions and Answers Cost N600 MTN recharge card.
ALSO SEE>>2018 Waec Gce Chemistry Practical Questions and Answers | Waec Gce Exam Runs
First Procedure on how to Subscribe
To subscribe for Chemistry Questions and Answers send the card pin, Subject name(Chemistry Objective and Essay Questions and answers ) and your phone number Via SMS or Via WhatsApp To 08065889844
ALSO SEE>>2018 Waec Gce CRS Objective and Essay Questions and Answers | Waec Gce Exam Expo
Note: 9Jatechs 2018 Waec Gce Chemistry Objective and EssayQuestions and Answers is N600 MTN Card
Note: please do not call our number, simply send message on whatsapp or using SMS
For More Info Whatsapp 08065889844
2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for .2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for Chemistry Objective and Essay .
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
Also see the 2018 Waec Gce Biology Practical Questions and Answers.
2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for .2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for Chemistry Objective and Essay .
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
ALSO SEE>>2018 Waec Gce Physical and Practical Geography Questions and Answers | Waec Gce Expo
2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for .2018 Waec Gce Chemistry Objective and Essay Questions and answers now available, To get all questions and answers for Chemistry Objective and Essay .
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).
9Jatechs deliver it answers at midnight to her subscribe. Expo for 2018 Waec Gce Chemistry Objective and Essay Questions and answers is also available on our portal (9Jatechs).